Regulatory
Wir beraten Sie zu allen nationalen und internationalen regulatorischen Themen, die im Rahmen der Arzneistoffentwicklung (Drug Substance, Drug Product) wichtig werden.
Diese Website verwendet Cookies
Notwendige Cookies helfen dabei, eine Webseite nutzbar zu machen, indem sie Grundfunktionen wie Seitennavigation und Zugriff auf sichere Bereiche der Webseite ermöglichen. Die Webseite kann ohne diese Cookies nicht richtig funktionieren.
| Name | Anbieter | Zweck | Ablauf |
|---|---|---|---|
| wire | cmc-pharma.de | Der Cookie ist für die sichere Anmeldung und die Erkennung von Spam oder Missbrauch der Webseite erforderlich. | Session |
| cmnstr | cmc-pharma.de | Speichert den Zustimmungsstatus des Benutzers für Cookies. | 1 Jahr |
Statistik-Cookies helfen Webseiten-Besitzern zu verstehen, wie Besucher mit Webseiten interagieren, indem Informationen anonym gesammelt und gemeldet werden.
| Name | Anbieter | Zweck | Ablauf |
|---|---|---|---|
| _ga | Registriert eine eindeutige ID, die verwendet wird, um statistische Daten dazu, wie der Besucher die Website nutzt, zu generieren. | 2 Jahre | |
| _gat | Wird von Google Analytics verwendet, um die Anforderungsrate einzuschränken. | 1 Tag | |
| _gid | Registriert eine eindeutige ID, die verwendet wird, um statistische Daten dazu, wie der Besucher die Website nutzt, zu generieren | 1 Tag |
Wir beraten Sie zu allen nationalen und internationalen regulatorischen Themen, die im Rahmen der Arzneistoffentwicklung (Drug Substance, Drug Product) wichtig werden.
Wir sprechen nicht nur darüber, sondern wir schreiben und verstehen was wir schreiben, denn wir sind wissenschaftlich ausgebildete „Technical Writer“ und erstellen „Drug Master Files, DMFs“, „Active Substance Master Files, ASMFs“, “Certificates of Suitability, CEPs“ „Investigational Medicinal Product Dossiers, IMPDs“, „Investigational New Drugs, INDs“ und weitere Zulassungsdokumente des Moduls 3 des „Common Technical Documents, CTD“.
Die hervorragenden Kenntnisse von Prozessen und Methoden des CMC-Teils helfen in sehr vielen Fällen die Dokumente ohne behördliche Rückfragen ins Ziel zu bringen. Wir sind hier unserem Firmennamen „Chemistry – Manufacturing and Controls, CMC“ verpflichtet und erstellen alle notwendigen Dokumente der Module 2.3 und 3 für regionale und internationale Zulassungen im eCTD Format „electronic Common Technical Document“.
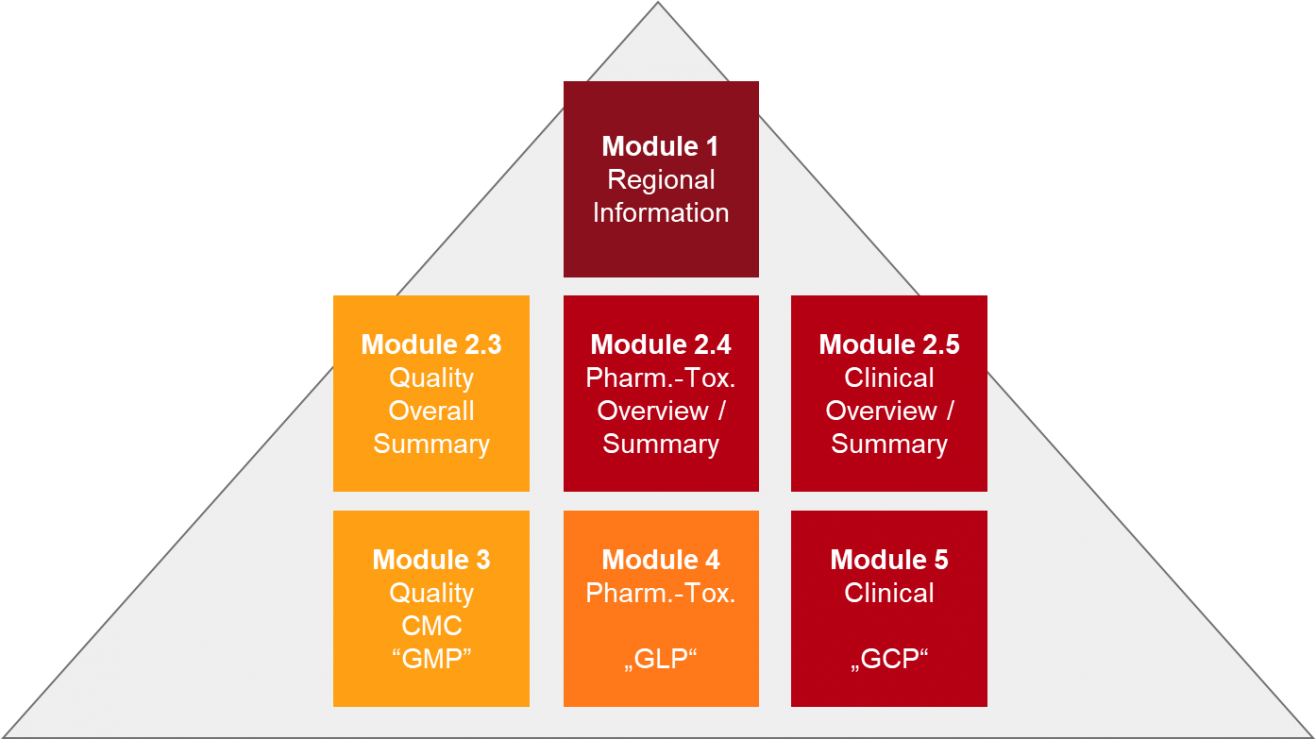
Struktur des electronic Common Technical Document (eCTD) nach ICH M4.
Die CMC Pharma GmbH erstellt und prüft alle notwendigen Dokumente für die Module 2.3 und 3.